barostim neo system
The Barostim Neo System is indicated for patients who have a regular heart rhythm are not candidates for cardiac resynchronization therapy and have a. The device uses electrical impulses to stimulate the nerve that regulates blood pressure inducing the blood vessels to relax.

Baroreflex Activation Therapy By The Rheos System And The Barostim Download Scientific Diagram
Project Type Neuromodulation system Developer.

. The Barostim neo system is a novel implantable device that activates the carotid baroreflex. 16 2019 PRNewswire CVRx Inc a private medical device company announced today that it has received Premarket Approval PMA from the United States Food and Drug Administration FDA to market its BAROSTIM NEO device for heart failure in the United. 32 to the medical management arm and 40 to the device arm 38 implanted 2 withdrawn.
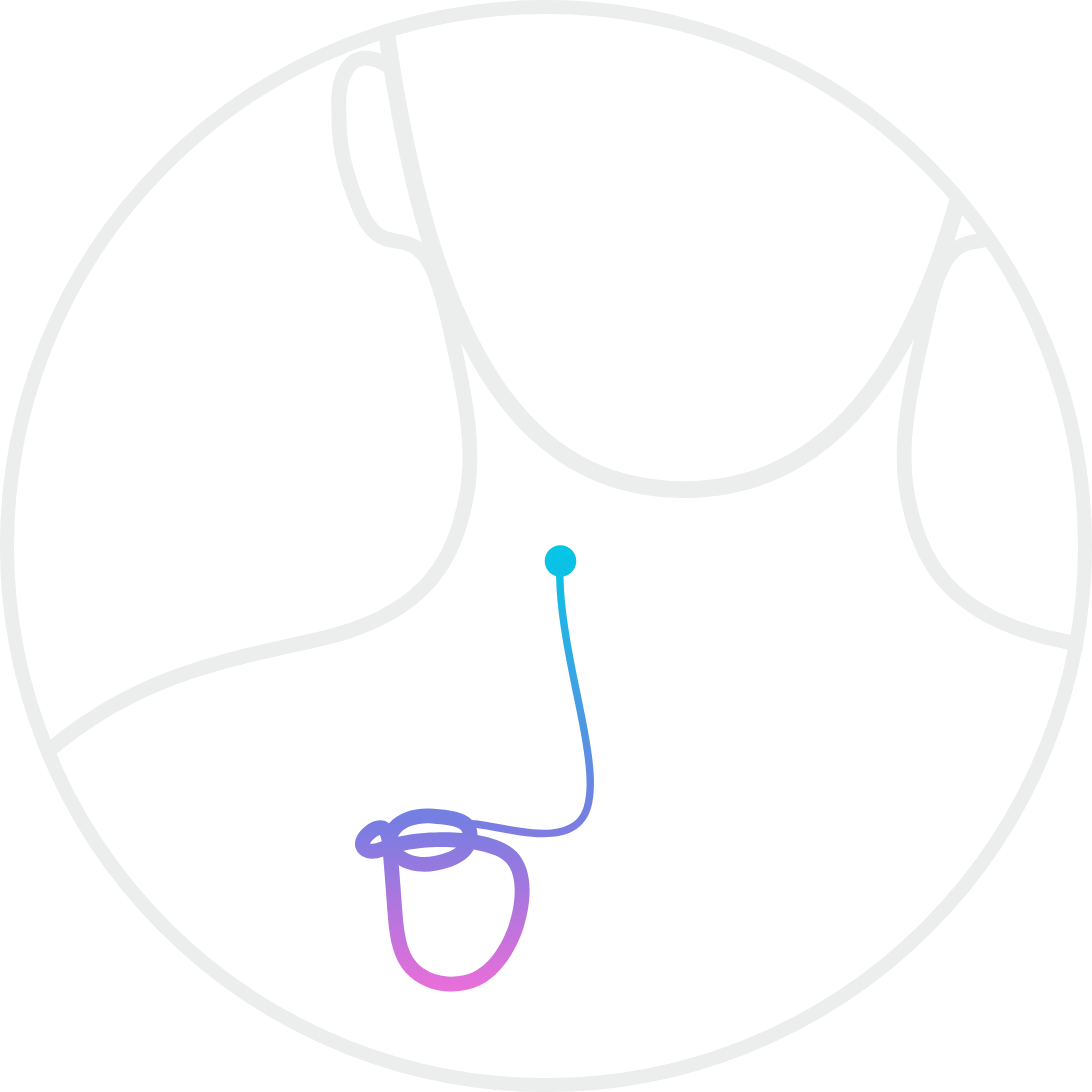
The BarostimNEO generator is inserted in a standard device pocket and these procedures typically take less than an hour usually performed on an outpatient basis. The BAROSTIM NEO System is designed to electrically activate the carotid baroreceptors the bodys natural cardiovascular regulation sensors. Learn More Patient stories Im able to do the things Ive always wanted to do -David Im back to walking three to four miles a day -Kevie.
REGISTER for free or LOG IN to view this content. Seventy two subjects were randomized. IPG Torque Wrench Port Plug 2102 Carotid Sinus Lead CSL Kit.
The therapy can be turned OFF by simply pressing a button to easily observe the difference Barostim neohas on blood pressure and other hemodynamic parameters. In the model Barostim reduced over a lifetime the rates of myocardial infarction by 19 stroke by 35 heart failure by 12 and end-stage renal disease by 23. Barostim was estimated to be cost-effective compared with optimal medical treatment with an incremental cost-effectiveness ratio of 7 797QALY.
In patients with resistant hypertension electrically activation. The Barostim System Baroreflex Activation Therapy is delivered by the BarostimNEO an implantable pulse generator IPG designed to deliver continuous electrical stimulation to carotid baroreceptors. More slides Presentation.
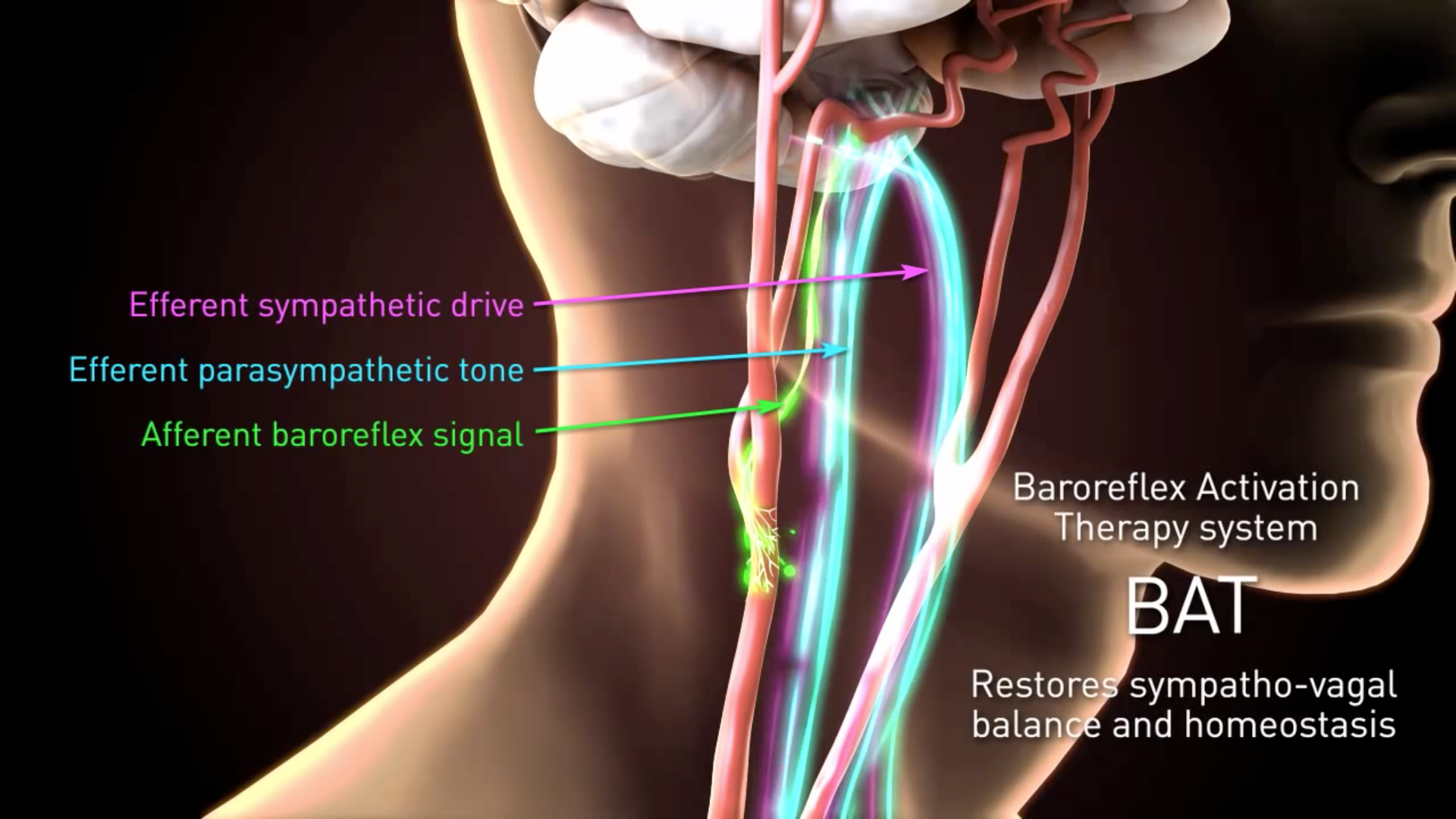
After a simple mapping procedure the Carotid Sinus Lead is sutured to the carotid sinus. The BAROSTIM NEO System Premarket Approval P180050 is a Class III carotid sinus stimulator an implantable medical device that delivers electrical signals to the bodys pressure sensors to. Res The brain works to counteract this perceived rise in blood.
When the baroreceptors are activated signals are se nt through neural pathways to the brain and interpreted as arise in blood p sure. The study closed to new enrollments on. Barostim neois a subcutaneous reversible treatment.
Lead Model 1036 or 1037 Implant Adapter Model 5033 Implant Tool Model 5031 1036 1037 Programmer System CPS. The mechanism of action is electrical stimulation of the baroreceptors which does not alter or destroy the structure of the baroreflex. Barostim is a novel Congestive Heart Failure CHF treatment that uses the power of the brain to improve symptoms like breathlessness fatigue and swelling.
Barostim is an FDA approved implantable device to treat people with CHF and a low ejection fraction systolic heart failure who do not qualify for Cardiac Resynchronization Therapy CRT or for whom CRT is not effective. When to apply a planned 2 stent technique and which to choose. Participants will be implanted with the BAROSTIM NEO System and will continue to receive optimal stable Guideline Directed Medical Therapy GDMT for heart failure American Heart Association AHA American College of Cardiology ACC guidelines including drugs to be determined by the subjects physician.
The Barostim System is implanted in a safe and straightforward surgical procedure. The BAROSTIM NEO System includes the following components. Parameters assessed during visits are.
The BAROSTIM NEO Hypertension Trial is a prospective randomized controlled trial assessing the safety and efficacy of the BAROSTIM NEO System in subjects with resistant hypertension. BAROSTIM NEO provides significant clinical benefit to heart failure patients MINNEAPOLIS Aug. Baroreflex Activation Therapy Barostim neo System Presenter.
Food and Drug Administration in 2019 following successful trials that were led by MUSC Health cardiologist Michael Zile MD. October 28 2013. The purpose of this registry NCT02880618 is to evaluate the effect of BAROSTIM THERAPY with the BAROSTIM NEO System in the commercial setting in subjects recently implanted under the CE-Marked indication for heart failure with reduced ejection fraction HFrEF.
Device Models Implantable Pulse Generator IPG. Anne Kroman Barostim won breakthrough device approval from the US. The minimally-invasive neosystem uses CVRx patented Barostim Therapy technology to trigger the bodys own natural systems by electrically activating the carotid baroreceptors the bodys natural cardiovascular regulation sensors.
The neosystem is the CVRx next generation system for improving cardiovascular function. ComputerSoftware Programmer Interface PI 9010. A prospective randomized study describing the safety and efficacy of the BAROSTIM NEO System in heart failure subjects with left ventricular ejection fraction equal to or less than 35 percent.
It decreases the sympathetic activity and inhibits the renin system which results in reduced blood pressure and heart rate. All subjects are now in long term follow-up and are required to have at least one annual visit. The system received CE mark from the National Standards Authority of Ireland NSAI in September 2014 to treat heart failure patients with an ejection fraction less than or equal to 35.
The Barostim System comprises the Barostim NEO IPG the Carotid Sinus Lead and a simple intuitive Programmer. Barostim Baroreflex Activation Therapy BAT uses the power of the autonomic nervous system to improve symptoms of heart failure. Office Cuff Blood Pressure.
Barostim Neo is a neuromodulation system developed by CVRx for the treatment of heart failure and hypertension. Barostim Neo - Baroreflex Activation Therapy for Heart Failure CMS Back to Approved IDE Studies Barostim Neo - Baroreflex Activation Therapy for Heart Failure Study Title Barostim Neo - Baroreflex Activation Therapy for Heart Failure Sponsor Name CVRx NCT Number NCT02627196 IDE Number G120010 CMS Approval Date 2016-06-23 Additional.
Cvrx S Barostim Neo Gets Ce Mark For Use With Mris Massdevice

First Rheos And Second Barostim Neo Generation Baroreflex Download Scientific Diagram

Barostim Neo Electrical Stimulator Approved For Heart Failure In Europe Video Medgadget

A Barostim Neo Electrode Assembly And Implantable Pulse Generator Download Scientific Diagram
First Implant Made For Barostim Neo Device To Treat Hypertension Daic

Barostim Baroreflex Activation Therapy Cvrx

Interventional Treatment For Heart Failure Receives Fda Approval Biba Medtech Insights
Barostim Baroreflex Activation Therapy Cvrx

Cvrx Barostim Neo Now Cleared For Mri Use In Europe Medaxs

Barostim Neo Neuromodulation Implantable System Usa

Innovative Device Therapie Der Herzinsuffizienz Management Krankenhaus
Barostim Neo 1 Radcliffe Cardiology

Fda Approves Device To Treat Patients With Heart Failure Wjar

Baroreflex Activation Therapy Barostim Neo System Tctmd Com

Barostim Neo Neuromodulation Implantable System Usa

Investigational Device For Heart Failure Patients Stimulates Cells In Arteries To Improve Function Youtube


Comments
Post a Comment